Imagine unearthing a baby woolly mammoth from the Siberian permafrost – its skin, hair, and even internal organs immaculately preserved after tens of thousands of years. Such discoveries spark a tantalizing question: could modern science rejuvenate ancient species from these frozen time capsules? Researchers and biotech startups are now pursuing that “Jurassic Park” dream, using cutting-edge techniques like cloning, CRISPR genome editing, stem cells, and selective breeding to bring Ice Age icons – from woolly mammoths to cave lions – back to life. This article explores, in rigorous detail, how de-extinction science is advancing, where these frozen specimens are found, the formidable technical challenges, real-world projects underway, and the profound ethical and ecological questions that arise.
We Will Talk About
Frozen Time Capsules: Permafrost Preservation of Ice Age Life
A remarkably well-preserved baby woolly mammoth, nicknamed Lyuba, was recovered from Siberian permafrost. Permafrost acts as nature’s freezer, protecting soft tissue (skin, muscle, even fur) and genetic material for tens of thousands of years.
Vast expanses of Arctic permafrost – permanently frozen ground in places like Siberia, Alaska, and Canada’s Yukon – serve as time capsules for Pleistocene wildlife. In these regions, animals that died during the Ice Age were sometimes buried in cold, oxygen-poor conditions that sharply slowed decay. The result is astonishing preservation: whole carcasses of mammoths, woolly rhinos, prehistoric horses, bison, and even cave lion cubs have been found with flesh and fur intact. For example, miners in Yukon recently uncovered a 30,000-year-old baby mammoth with her trunk, ears, and tail almost perfectly preserved – “she appeared as though she had just passed away,” according to the paleontologist who examined her (The Archaeologist). In Siberia, local tusk hunters searching the melting permafrost have stumbled on treasures like a 28,000-year-old cave lion cub so well mummified that you can still make out every one of her whiskers. Even internal organs can remain intact inside these frozen mummies.
Permafrost preserves such specimens by locking them in cold storage. Subzero temperatures arrest microbial activity and enzymatic decay, and in some cases, natural “freeze-drying” (desiccation) occurs, turning tissue into a stable, dry state. DNA can also survive for remarkably long periods: in 2021, scientists even recovered fragments of million-year-old mammoth DNA from permafrost-preserved teeth. At the same time, DNA does break down over time, but the cold, dry, dark conditions under the tundra slow that process to a crawl. Permafrost is essentially a giant freezer, guarding “sensitive data like DNA as well as soft tissue like muscle, skin, and hair” across millennia. This exceptional preservation gives hope that intact genetic material – or even whole cells – might be retrieved from long-extinct species.
Scientists have coaxed surprising activity from permafrost finds. In a landmark 2019 experiment, Japanese researchers extracted nuclei from the 28,000-year-old frozen muscle of a mammoth named “Yuka” and implanted them into mouse egg cells. Under the microscope, they observed a tantalizing sign of life: the ancient mammoth DNA began to recruit spindle proteins and partially form chromosomes, as if preparing for cell division. While no living embryo came of it, the study demonstrated that some cellular structures can survive eons and respond when placed in a modern cellular environment. As the team hailed, it was “the first time in the world” that biological activity was observed in mammoth fossils, showing that well-preserved frozen cells retain partially viable nuclei. This breakthrough raises the question: might a whole organism be revived if enough intact DNA or cells are found?
The Best Hunting Grounds for Frozen Fossils
The most spectacular frozen fossils have emerged from permafrost in Siberia’s Yakutia region (the Sakha Republic) and in North America’s far north. In these polar regions, permafrost extends tens or hundreds of meters deep, creating a cemetery of extinct creatures from the last Ice Age. Siberia’s thawing tundra has yielded not only mammoths and lions but woolly rhinoceroses, steppe bison, ancient wolves, and prehistoric horses, often discovered by accident during industrial activities or by locals hunting for Pleistocene ivory. In one notable case, a 42,000-year-old foal (baby horse) was found in Yakutia’s Batagaika crater, astonishingly containing liquid blood and urine when thawed. Scientists extracted the blood from this Ice Age foal – the oldest liquid blood on record – and were eager to attempt cloning the extinct Lenskaya horse species to which it belonged. They made over 20 attempts to grow viable cells from the foal’s tissues, albeit without success so far. Nevertheless, the discovery attests to the unparalleled preservation that permafrost can offer. (For context, only one other prehistoric creature – a 15,000-year-old mammoth carcass – has ever yielded liquid blood, underlining how extraordinary the foal find was.)
Siberia’s permafrost, in particular, has become a paleontological goldmine as climate warming and human activities expose once-buried carcasses. Usually, dead animals decompose or get scavenged, but those that ended up in freezing mud or ice can emerge remarkably unchanged in appearance. The two cave lion cubs discovered in recent years, nicknamed Sparta and Boris, illustrate this well. Sparta, found in 2018, is “among the best preserved Ice Age animals ever uncovered,” with skin, teeth, and even whiskers intact after ~28,000 years in the permafrost. Boris, discovered nearby, is older (~43,000 years) but still retains fur and tissue. These cubs look “remarkably similar to modern lions” except for subtle Ice Age adaptations like thicker fur—discoveries like these fuel scientific excitement – and public imagination – about actually recreating these animals. As Albert Protopopov, a Yakutian paleontologist involved in the lion finds, remarked: “There is a very realistic chance to recreate cave lions, and it would be a lot easier than to clone a woolly mammoth”. His team even speculated that cave lions could potentially be cloned using modern lion surrogates, given the superb quality of the frozen cubs’ DNA and their close relation to living big cats.
But retrieving intact genetic material is only the first step on the long road to de-extinction. How exactly do scientists plan to turn ancient DNA into a living, breathing animal?
The Science of Species Revival: Cloning, CRISPR, and More
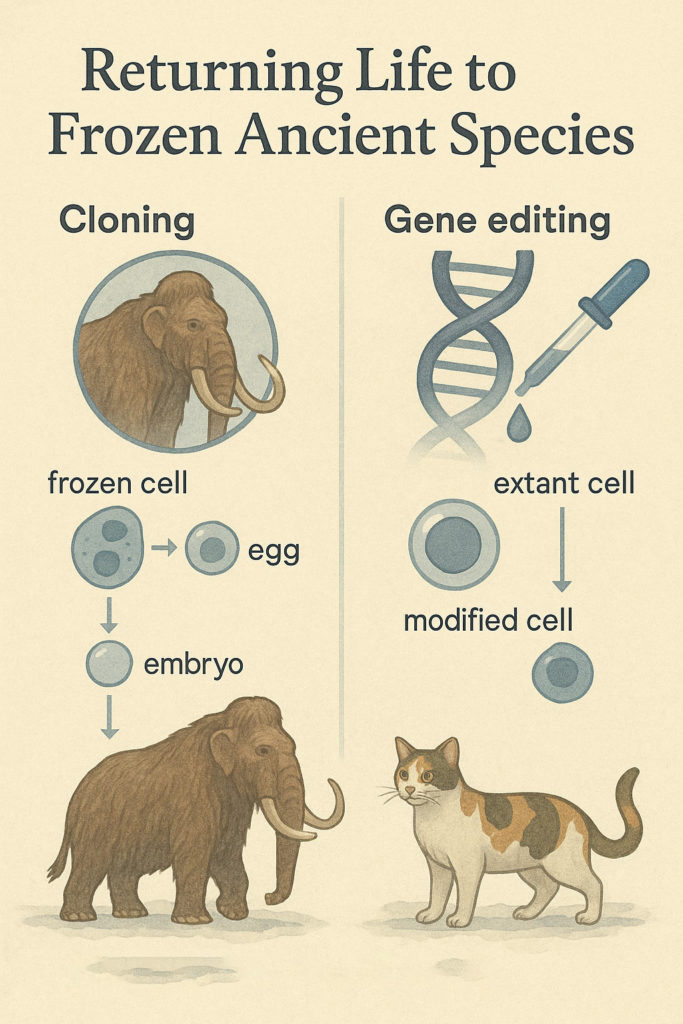
Bringing back an extinct species is an immensely complex task that can draw on several advanced biological techniques. Broadly, de-extinction efforts fall into a few overlapping approaches:
Cloning from preserved cells (Somatic Cell Nuclear Transfer)
If you have an intact cell (or at least a nucleus) of the extinct animal, you can attempt to clone it by inserting that nucleus into an egg of a closely related species.
The egg, prompted by the nuclear DNA, can develop into an embryo that is a genetic clone of the extinct animal. This is the same technique used to create Dolly the sheep, the first cloned mammal, in 1996. In the context of extinct species, the most famous example is the Pyrenean ibex (bucardo) – the only creature so far to be brought back from extinction (albeit briefly) via cloning. Scientists in Spain had preserved skin cells of the last bucardo before it died in 2000; using domestic goats as surrogates, they cloned a female bucardo in 2003. That clone sadly died minutes after birth due to lung defects (cloning often has a high failure rate), but it marked “the first extinct animal to undergo de-extinction” and proved the concept. The bucardo project underscored both the promise and pitfalls of cloning: you can resurrect lost species if viable cells are available, but success may require hundreds of embryo attempts, and even then, resulting offspring may have health problems (National Geographic). For Ice Age giants, true cloning would require a nucleus from a frozen cell – a long shot, since most permafrost specimens have only fragmented DNA. Still, as described earlier, partial nuclear structures have been teased into activity (e.g., Yuka’s nuclei in mouse eggs). Researchers in Russia and South Korea have been actively hunting for well-preserved cells in mammoths and other fossils with the hope of cloning. Dr. Hwang Woo-Suk, a South Korean cloning pioneer, has an agreement with Yakutian scientists and has received tissue from 12,000-year-old cave lion cubs, which he believes is “enough to clone the extinct cave lion species”. Hwang’s team (in collaboration with Russia’s North-Eastern Federal University) is likewise searching for a viable mammoth cell, though none has been found yet. Cloning attempts on such ancient material face unprecedented hurdles – but if a miracle cell is discovered, the Dolly playbook could be applied.
Genetic Engineering and CRISPR Genome Editing
In practice, no Ice Age fossil has yielded a perfectly intact genome in one nucleus.
What scientists do have are sequenced genomes reconstructed from ancient DNA fragments. For example, multiple woolly mammoth genomes have been sequenced by assembling billions of DNA snippets from bone and hair samples. These data allow us to compare an extinct genome to that of a living relative – e.g., mammoth vs. Asian elephant – and pinpoint the genetic differences that made a mammoth a mammoth. Using CRISPR-Cas9 gene editing, we can take living cells of the relative and edit them to carry the extinct creature’s DNA variants. This is the strategy of Harvard geneticist George Church and the startup Colossal Biosciences for the woolly mammoth project. “We’re not cloning a mammoth because we have no living cell,” explains evolutionary biologist Beth Shapiro, “What we actually mean by de-extinction is using the tools of genome engineering to resurrect the core traits of these species”. In other words, the goal is to create an Asian elephant that is genetically and physiologically akin to a woolly mammoth, by splicing in mammoth genes for cold-resistance (thick hair, insulating fat, etc.). To do this, scientists first identify key genomic differences: “We line up several mammoth genomes and elephant genomes and ask, where are all mammoths the same and different from elephants? Those are candidates for mammoth-specific traits,” Shapiro says. Church’s team has indeed catalogued dozens of important mutations – in genes for mammoth hair, subcutaneous fat, hemoglobin, and more – and used CRISPR to edit many of them into elephant cells in the lab. Early on, they reported successfully swapping 14 gene loci in elephant skin cells to mammoth versions as a proof of concept. The plan is to keep stacking more edits; current estimates are that 50–60 edits may capture the main mammoth traits out of the 1.4 million DNA differences between the species. Colossal Biosciences, which has raised over $75 million for de-extinction, ambitiously claims it is “already in the process of the de-extinction of the woolly mammoth”, having collected DNA samples and begun gene editing in its labs (Popular Mechanics). The company’s stated goal is to produce mammoth-elephant hybrid embryos by 2027 and ultimately birth live mammoth-like calves. Such embryos would likely be gestated in an elephant surrogate mother (or perhaps an artificial womb, as discussed later) and, if all goes perfectly, result in a creature that looks and behaves like a woolly mammoth. This approach – sometimes dubbed “proxy de-extinction” – does not revive a species exactly as it was, but creates a new, genetically engineered version that should closely resemble the extinct species. Besides the mammoth, similar genome-engineering projects are targeting the passenger pigeon (extinct 1914, using band-tailed pigeon cells) and the Tasmanian tiger or thylacine (extinct 1936, using dunnart or Tasmanian devil cells), among others. CRISPR technology is thus a powerful tool to “build” an extinct genome in a living cell. However, a major challenge remains: turning that edited cell into a living animal, which leads to…
Stem Cell Technology and Assisted Reproduction
Even if we edit a cell to have an extinct genome, we need to develop it into an embryo.
This is where advanced reproductive biology comes in. Scientists can attempt somatic cell nuclear transfer with an edited cell (essentially cloning, using the edited cell’s nucleus in a donor egg). Alternatively, they may use induced pluripotent stem cells (iPSCs) – reprogramming adult cells into embryonic-like stem cells – and then guide those iPSCs to form eggs or sperm. The iPSC route is being explored for species conservation generally (e.g., creating gametes for nearly extinct rhinos), and it could be pivotal for de-extinction. Notably, Colossal reported in 2022 that it managed to reprogram Asian elephant cells into a stem-cell state (The Nature), a first for that species. These elephant iPSCs could potentially be coaxed to form embryos or germ cells, which then undergo CRISPR edits for mammoth traits. The process might involve creating blastoids or embryonic structures from iPSCs and implanting those into a surrogate. It’s bleeding-edge science – no one has yet made viable egg or sperm cells from elephant iPSCs – but progress is being made (similar work in mice has produced fertile eggs from stem cells). Another approach is to inject donor primordial germ cells into embryos of a host species. For example, the nonprofit Revive & Restore is working on the passenger pigeon by editing band-tailed pigeon germ-line cells and inserting them into developing pigeon embryos, hoping the resulting adult birds will produce eggs/sperm carrying passenger pigeon genes. This method bypasses the need for a full surrogate pregnancy in a larger animal, but it’s only feasible for species that can be bred via eggs (birds, fish, etc.). In mammals, the prevailing pathway is still to implant an embryo into a closely related species’ uterus. Thus, mastering techniques like interspecies embryo transfer, artificial insemination, or even artificial wombs is crucial. One remarkable case on the reproductive frontier is the northern white rhino – functionally extinct with only two females left. Scientists are creating rhino embryos via IVF using preserved cells and southern white rhino surrogates. This isn’t de-extinction (the species isn’t fully gone yet), but it parallels what would be needed for, say, a woolly mammoth: lab-created embryos, surrogate mothers of another species, and possibly even in vitro gestation systems.
Back-Breeding (Selective Breeding for Ancient Traits)
An older-fashioned approach to “recreating” an extinct animal is to selectively breed its closest living relatives to express the traits of the extinct form.
This doesn’t truly resurrect the extinct genome, but it can produce an analog animal that looks or behaves like the lost species. This method has been used for species that died out more recently and have direct descendants. A classic example is the aurochs, the wild ancestor of domestic cattle (extinct 1627). Several projects in Europe (e.g., the Tauros Programme and others) are back-breeding cattle to emphasize aurochs-like features – large size, certain horn shape, color, and hardiness. Over successive generations, they aim to produce a “near-aurochs” that could fill the aurochs’ ecological role as a wild grazer. Similarly, the Quagga, a zebra subspecies with partial striping (extinct 1883), is being recreated by selectively breeding plains zebras that have reduced striping; the Quagga Project in South Africa has already bred zebras that closely resemble historical quaggas. While back-breeding can restore an extinct appearance or niche, it only works if the genetic variability for the needed traits still exists in the living gene pool. It’s not an option for woolly mammoths (elephants lack most mammoth-specific genes) or cave lions (you can’t really breed a modern lion to grow thick underfur and other unknown traits without genetic engineering). However, for Ice Age horses or bison, one could breed existing populations to highlight latent ancient traits (e.g., the hearty, shaggy “steppe bison” look). Back-breeding is thus a complementary strategy, often used in concert with genetic data from ancient DNA to guide which traits to target. It is generally less precise than molecular methods, but it has the advantage of working in vivo with natural reproduction.
Each of these approaches can also be combined. For instance, one could use CRISPR on iPS cells and then clone the edited cells via nuclear transfer. Or back-breeding could be informed by genome sequencing of museum specimens. In practice, de-extinction projects use multiple tools.
From Lab to Field: Current De-Extinction Projects and Examples
Around the world, a handful of ambitious projects are already underway to apply these techniques to extinct species – including ones frozen in permafrost:
Woolly Mammoth Revival
The effort to bring back the woolly mammoth (or rather, a mammoth-like elephant) is arguably the most high-profile de-extinction project. Spearheaded in academia by George Church’s lab at Harvard and boosted by Colossal Biosciences, the project’s goal is to create a cold-resistant Asian elephant that can thrive in Arctic climates. Rather than cloning a mammoth from a carcass, the team is inserting mammoth genetic traits into elephant DNA. As Church clarifies, “People say we’re de-extincting mammoths… We’re not. We’re trying to de-extinct genes” – essentially engineering an elephant-mammoth hybrid. Dozens of mammoth genes have been identified, and experiments are ongoing to edit these into elephant cells. In 2021, Colossal announced a $15 million launch and aims to have mammoth-elephant calves in 4–6 years. By 2023, they raised an additional $60 million and boldly stated the first calves could be born by 2027 (Popular Mechanics). (Whether this timeline is realistic remains to be seen.) Colossal’s plan involves either implanting embryos into elephant surrogates or developing an artificial womb for gestation. Using live elephants raises ethical concerns – Asian elephants are endangered and pregnancy is risky – so Church’s lab has been exploring incubating embryos ex utero (they’ve tested the concept with mouse embryos). If successful, the “mammoth” (technically a proxy species sometimes nicknamed a mammophant) would be introduced into large fenced reserves in the Arctic, such as Pleistocene Park in Siberia run by ecologists Sergey and Nikita Zimov. The scientific rationale is that herds of mammoth-like elephants could help restore Ice Age tundra grasslands and even slow permafrost melt by trampling insulating snow. “Moving genetically adapted elephants to the Arctic offers an opportunity to sequester carbon and prevent permafrost from thawing,” Church argues, linking de-extinction to climate mitigation. This vision is inspiring, though it remains a long-term prospect. As one genetics expert noted, “The public should not leap to the conclusion we are on the edge of cloning woolly mammoths or dinosaurs” – even constructing an embryo is a massive challenge, and finding a willing (and safe) mother elephant is another. Nonetheless, the mammoth project is forging ahead, generating valuable techniques in genome editing and assisted reproduction.
Cave Lion (Panthera spelaea)
The discovery of exceptionally preserved cave lion cubs in Siberia has opened the door for possibly resurrecting these Pleistocene big cats. Russian scientist Dr. Albert Protopopov has expressed optimism due to the lion’s close relation to modern lions: “easier [to recreate] than a mammoth,” he says. The Yakutian academy provided samples of the frozen cubs to Hwang Woo-Suk’s team in South Korea, which specializes in cloning. By 2016, Hwang’s lab had received muscle and skin from two cubs and declared its intent to attempt cloning a cave lion. There was some controversy over samples (the Yakutian side was cautious, only sending small pieces), but Hwang believed it was sufficient to proceed. No clone has been announced yet – likely because, as with mammoths, intact nuclei are hard to find – but efforts continue. If a cloned embryo were created, it could potentially be implanted into an African lioness (the cave lion was either a close cousin or subspecies of the modern lion). Protopopov even suggested that any gaps in the ancient DNA might be filled by splicing in genes from living lions, essentially producing a lion-cave lion hybrid. In popular media, there have been reports that scientists “are about to” clone the frozen cave lions, though this remains aspirational until viable cells are confirmed. Still, the cave lion is a prime candidate because its extinction (~13,000 years ago) is recent, its DNA is relatively intact, and a surrogate species (Panthera leo) is available. Should a cave lion be born, it would be a sensation – and would raise questions about where such apex predators might live (perhaps in large wildlife reserves).
Woolly Rhinoceros
Another Ice Age giant found in permafrost, the woolly rhino (Coelodonta antiquitatis) has had its genome sequenced from frozen remains. While no explicit de-extinction project is public, the techniques developed for the mammoth could, in theory, be applied to a rhino using a white or black rhino surrogate. That said, modern rhinos are endangered themselves, making any attempt ethically fraught. A perfectly preserved woolly rhino calf named Sasha was found in Siberia in 2014 with strawberry blond fur still attached; such finds make scientists ponder revival, but concrete efforts are limited compared to mammoth or thylacine projects.
“Proxy” Projects (Non-permafrost species)
In addition to Ice Age mammals, several projects are focused on species that went extinct more recently (and typically were preserved via museum specimens or DNA kept in labs). For example, Revive & Restore is working on the Passenger Pigeon using CRISPR on band-tailed pigeon cells – an effort that involves harvesting primordial germ cells from pigeon eggs and editing in passenger pigeon gene variants, with the goal of hatching chicks that behave like passenger pigeons. Similarly, a team in Australia (in partnership with Colossal) is pursuing the Tasmanian Tiger (Thylacine) de-extinction. They have the thylacine genome from preserved pelts and are exploring ways to create thylacine embryos using stem cells from a small marsupial called a dunnart as the “canvas” for genome editing. Colossal also announced in 2023 a project to bring back the dodo, leveraging the genome that Beth Shapiro’s team sequenced from historical dodo remains. These efforts, while not based on permafrost specimens, use many of the same technologies and often share the ultimate goal of rewilding the animals into their former habitats (or analog habitats). Each provides a test case that can inform the others. For instance, progress in growing marsupial embryos in vitro for the thylacine could help in developing artificial wombs for placental mammals like mammoths; the passenger pigeon project’s advances in germ-line engineering in birds could be transferable if one ever attempted to resurrect, say, the great auk or other extinct birds.
What is clear from all these endeavors is that de-extinction is no longer science fiction – it’s a burgeoning scientific enterprise, attracting top talent and significant funding. However, success is far from guaranteed. As we’ll see next, the challenges to create a healthy, living animal from ancient DNA are monumental.
The Challenges: Decoding Death and Rebuilding Life
For all the excitement, de-extinction scientists are quick to emphasize just how hard this work is. Biology has many failure points, and bringing back an Ice Age species involves threading the needle through all of them:
DNA Quality and Completeness
Once an organism dies, its DNA begins to degrade into smaller and smaller fragments. Ancient DNA is often chemically modified and chopped up by microbes or water. Even in permafrost, time is an enemy of DNA integrity. Mammoth DNA recovered from fossils may come in millions of pieces, not a tidy set of chromosomes. While advanced sequencing can read these fragments and even overlap them to assemble a near-complete genome, that’s only the digital part of the solution. We cannot yet synthesize an entire mammalian genome from scratch and properly fold it into chromosomes (billions of base pairs long) and inject those into an empty nucleus. So, if no intact cell nucleus is available, scientists must piggyback on a living genome (as with CRISPR editing of an elephant cell). “To clone a mammoth, you need an intact nuclear genome of a mammoth, and that just doesn’t exist,” Beth Shapiro explains bluntly. She and others caution that we will never get 100% of the original genome; at best, we create a close approximation. In practice, an edited hybrid will have some DNA of the extinct creature (targeted genes) and the rest from the living relative. This may be sufficient for the phenotype, but it’s not a true genetic clone of the extinct animal.
Finding Viable Cells
The holy grail would be a viable reproductive cell (sperm or egg) or a totipotent embryonic cell from a frozen specimen. To date, none has been found for Ice Age animals. The closest successes involve much more recent extinctions where tissues were preserved. For instance, the Bucardo clone was possible because scientists had kept a piece of skin frozen at – 100°C for just 3 years. In another case, scientists cloned a mouse from another mouse that had been frozen (at –70°C) for 16 years – but that was a laboratory preservation, not an ancient wild fossil. In permafrost specimens, cells are often burst by ice crystals or turned into a kind of cellular jerky. The Japanese attempt with Yuka’s cells showed that some nuclear structures can be recovered, but also illustrated how far they are from true viability. The Yakutsk-Korean team searching for intact cells in the 42,000-year-old foal attempted dozens of cell cultures with no luck. As Dr. Semyon Grigoryev (head of Yakutia’s Mammoth Museum) conceded, even the foal’s beautifully preserved body is “absolutely hopeless for cloning purposes” if only blood cells are found – because adult mammalian red blood cells lack nuclei (and thus DNA). He said they are “trying to find intact cells in muscle tissue and internal organs” of that foal instead. This underscores the challenge: you might have an animal that looks pristine on the outside, but at the microscopic level, its cells are ruined. Solving this might require searching hundreds of carcasses in hopes that one died in just the right conditions (e.g., super-quick freeze) to preserve cellular integrity. Or, alternatively, advancing nuclear transplantation technology to reassemble a viable nucleus from damaged pieces – a feat currently beyond biology.
Embryo Development and Surrogate Mothers
Let’s suppose scientists create a fertilized embryo (either by cloning, IVF, or CRISPR). The next hurdle is getting that embryo to term. Implantation into a surrogate womb is delicate and failure-prone. Even with cloning of living species, success rates are a few percent at best – Dolly was the lone success out of 277 attempts. In the bucardo project, 208 cloned embryos yielded just 7 pregnancies and 1 birth, which then died. For a hybrid mammoth-elephant embryo, the unknowns multiply: Will the altered embryo send the right hormonal signals to trigger pregnancy in an elephant? Will the elephant’s immune system tolerate this partially foreign fetus? Gestation length might differ from a normal elephant’s (mammoths may have had a similar ~22-month gestation, but we don’t know for sure). Large mammals have long pregnancies, so each attempt ties up a surrogate for nearly two years, and any miscarriage or complication is a huge setback. Moreover, using surrogates of an endangered species (like Asian elephants, which number only ~50,000) raises ethical issues of risking the mother’s health (elephant births can be dangerous even in normal circumstances). George Church has acknowledged this concern: “If [Asian elephants] go extinct, we don’t want to have any role in it”, he says, hence the interest in artificial wombs to avoid using a rare elephant as a surrogate. An artificial womb for a mammoth is an active research topic – for instance, a team in Japan is developing extracorporeal uteruses for goats, and lamb fetuses have been sustained in artificial placenta bags for a short period. But growing a 100+ kg elephant fetus outside a living mother has never been done. Church’s plan is to “debug the process in mice and then transfer it over” to larger animals. Until such technology matures, de-extinction projects may have to use closely related species as surrogates: e.g., African elephants for mammoth hybrids (they’re slightly larger than Asian elephants), or lions for cave lions, or zebras for quaggas. Even for birds like the passenger pigeon, surrogate parenting is tricky – one strategy is to have domestic pigeons foster the chicks of the edited eggs, but will they recognize and feed a chick that might behave differently? Each species presents unique reproductive challenges. And after birth, the newborn must survive and thrive, which cloned animals sometimes struggle with due to immune deficiencies or developmental anomalies. The first bucardo clone, for example, had malformed lungs and lived only minutes.
Raising and Integrating the Species
Suppose a healthy extinct animal is born – what next? Many species are social and learn behaviors from their parents. A lone mammoth calf raised by humans (or by an elephant foster mother) might not learn how to find food, migrate, or interact with its own kind (since there are no others of its kind). For any de-extinct species, scientists must plan for proper socialization and enrichment, possibly by having multiple calves so they can form a cohort. This means the project’s aim is not just for one individual but for a breeding population, which exponentially increases the effort. Genetic diversity is a serious concern here: if all clones stem from one or two source genomes, the resulting population will be inbred and vulnerable. “Generating just one or a few animals via cloning will not produce a viable population,” as conservation biologist Bill Holt warned – even if healthy, they’d lack diversity and be “very susceptible to disease or even climatic change” (National Geographic). Ideally, dozens of distinct cell lines or genome sequences would feed into the de-extinction to capture a range of genetic variation. For extinct species, this means sequencing many individuals’ remains (e.g., mammoth genomes from different regions and times) and, if possible, using CRISPR to mix and match alleles to increase heterozygosity. It’s a tall order, and no current project is near achieving it. Revive & Restore’s passenger pigeon plan involves blending genes from multiple museum specimens to avoid a clone of just “Martha” (the last pigeon) – a strategy others will likely adopt where DNA allows.
Ecological Unknowns
The revived species must be able to eat and survive in the modern environment. Flora and climate have changed since the Ice Age. Will a woolly mammoth find sufficient roughage on today’s tundra? (Proponents say yes, that Siberia and the Arctic have plenty of grass if kept trampled; skeptics note the ecosystem is not identical to the Pleistocene steppe). How will living elephants react to a hairy newcomer if they end up in the same sanctuaries? Similar questions apply to predators: a cave lion reintroduced to Siberia might compete with Siberian tigers or prey on endangered herd animals. These ecological factors mean any release has to be carefully managed. Initially, most de-extinct animals would likely live in semi-wild enclosures for observation. There’s also the possibility of unforeseen health issues: pathogens in the environment that did not exist in the past, or conversely, dormant microbes within the resurrected animal to which modern species have no immunity (though this latter risk is speculative mainly and more relevant to ancient microbes than animals).
The road to resurrection is arduous. As Dr. David Wildt of the Smithsonian cautioned when the bucardo was cloned: “This isn’t a sign we’re on the edge of Jurassic Park. Even if you can construct embryos, there are no appropriate surrogate mothers for long-dead species”. That fundamental mismatch – trying to fit an Ice Age square peg into a 21st-century round hole – is what makes de-extinction so challenging. Yet, step by step, scientists are pushing the boundaries. Each technical milestone (e.g., recovering ancient chromosomes, editing elephant stem cells, or cloning an endangered modern species) brings the vision closer.
Ethics, Risks, and the De-Extinction Debate
Resurrecting extinct species is not just a scientific quest – it’s a venture rife with ethical dilemmas and philosophical questions. As we inch toward the first de-extinct birth, experts from bioethicists to ecologists are weighing the potential benefits and harms:
Animal Welfare and Ethics of the Individual
One of the foremost concerns is the welfare of the de-extinct creatures and their surrogates. Cloning and cross-species reproduction can involve a lot of failures and suffering – miscarriages, deformities, and early deaths are common in cloning experiments. Is it ethically acceptable to create potentially short-lived or unhealthy animals in the name of science? Ian Wilmut, the creator of Dolly, has said, “It should be done as long as we can provide great care for the animal. If there are reasonable prospects of them being healthy, we should do it. We can learn a lot about them”. In other words, if we can ensure a cloned mammoth or passenger pigeon has a decent quality of life, the endeavor might be justified for the knowledge gained and conservation tools developed. However, critics argue that these resurrected individuals could suffer just by being out of their natural context – e.g., a herd animal with no herd, a predator with no mother to teach it hunting. We also must consider the surrogate mothers: using an endangered elephant as essentially a lab vessel for a hybrid fetus raises questions of consent and cruelty. George Church’s push for artificial wombs is partly to mitigate this: “That’s not something you want to mess with… we don’t want to have any role in [elephants] going extinct” by harming live elephants in the process. The welfare discussion extends to once the creature is born – will it live in a zoo? A Pleistocene Park penned enclosure? Or roam wild and free (with all the dangers that entail)? Ensuring humane treatment and minimal suffering at all stages is a paramount ethical requirement voiced by scientists.
Playing God and Naturalness
On a philosophical level, some view de-extinction as hubristic meddling with nature. The sentiment that extinction is final – “dead means dead” – is deeply ingrained. “Extinction is an irreversible event… the idea you can reverse it is anathema, biologically but also philosophically,” says ecologist Prof. Dieter Hochuli. From this perspective, resurrecting species violates the natural order, akin to “playing God.” Detractors worry it signals that we see nature as a Lego set we can dismantle and reassemble at will. Proponents counter that humans have already been playing God, but mostly in destructive ways – wiping out species and altering the climate. Now, they argue, we have a chance to be creators and restorers. As conservationist Stewart Brand famously said, “We are as gods and have to get good at it.” Revive & Restore’s philosophy is that using biotechnologies for conservation is an extension of our responsibility. Advocates like philosopher Julian Savulescu suggest that human influence can “now be constructive… through genetic engineering, synthetic biology, and other technologies” to preserve and restore species. In essence, if we have the power to bring a species back, do we have a moral obligation to do so, especially in cases where humans caused the extinction (e.g., the dodo or passenger pigeon)? Some say yes – it’s justice for past wrongs. Others fear it reflects a dangerous technological arrogance and lack of humility toward natural processes.
Conservation Distraction and Resource Allocation
Perhaps the most pressing criticism from ecologists is that de-extinction could divert attention and funds from saving living species. We are in a biodiversity crisis with thousands of extant animals and plants verging on extinction. Every dollar and hour spent trying to resurrect a mammoth is one not spent on preventing elephants or polar bears from dying out. As one essay bluntly titled it, de-extinction can be a “fascinating but dumb idea” if it “takes resources away from saving endangered species and habitats”. While de-extinction proponents claim it’s not a zero-sum game – that we can do both – critics like Hochuli point out that in reality, conservation budgets are limited and choices are made. He calls it “greenwashing” to claim de-extinction helps conservation in the present. For example, Colossal markets itself as an “eco-restoration” company, but skeptics note that much of its work is focused on headline-grabbing extinct animals rather than directly rescuing endangered species (though Colossal does spin off some tech, like a project editing Asian elephants for disease resistance). The worry is a public perception issue: if people think we can ‘undo’ extinctions, they may become less motivated to support traditional conservation or to reduce destructive behaviors. It could foster complacency – a sense that extinction isn’t forever, so maybe habitat loss or poaching aren’t so bad because science can fix it later. Conservationists emphasize that preventing extinctions should be the priority, as “reintroductions have a high failure rate” and can’t truly recreate a lost ecosystem. There’s also the pragmatic point that a revived species will require extensive habitat and protection, the same as or more than an endangered species does.
Ecosystem Impacts and Invasiveness
What happens if we release a de-extinct species into today’s ecosystems? This is both exciting and worrisome. On the one hand, rewilding lost keystone species could help restore ecological processes. For instance, mammoths (or their proxy) might transform the Siberian tundra by knocking down trees and compacting snow, which could promote grasslands and keep permafrost colder. Some scientists theorize this could help slow climate change by preventing permafrost carbon release. Similarly, bringing back the passenger pigeon – once a primary seed disperser – could influence forest composition in eastern North America (though those forests have also changed in its absence). However, introducing any large animal has unpredictable effects. Could a population of mammoth elephants damage the fragile Arctic in unforeseen ways or conflict with human activities? If a carnivorous species like the cave lion were reintroduced, we must ensure it doesn’t wreak havoc on present-day fauna or livestock. It’s essentially an assisted migration across time – and as with moving species geographically, it can fail or have unintended consequences. Ecologist Joseph Bennett famously argued that “De-extinction could lead to biodiversity loss” if a revived species becomes invasive or simply drains resources that would’ve gone to other conservation efforts. That said, many de-extinction proposals involve contained or closely managed release sites (e.g., Pleistocene Park’s fenced area). The ethics here revolve around our duty to existing ecosystems versus the desire to restore past ones. For species that have no habitat left (e.g., a mammoth can’t roam in a densely populated world freely), is it right to bring them back only to live in quasi-zoo conditions?
Biosecurity and “Pandora’s Box” Fears
A more sci-fi set of concerns involves ancient pathogens. Could de-extinct animals carry old diseases that make a comeback? The permafrost is known to preserve microbes and viruses; there have been real cases like anthrax spores from a thawed reindeer carcass infecting people and reindeer in Siberia in 2016. Scientists have also intentionally revived “zombie viruses” from permafrost to study them, demonstrating that even 30,000- to 48,000-year-old viruses can infect hosts (fortunately, only amoebas in those studies). While an extinct mammoth itself likely wouldn’t harbor a live 30,000-year-old virus (any germs in its corpse would also be ancient and mostly inert), the process of digging up carcasses or thawing environments could expose humans to pathogens. Moreover, bringing back, say, a woolly mammoth means re-introducing its unique parasites and microbiome by extension – although realistically, those specific parasites are probably long gone or would have to be co-revived. Nonetheless, biosecurity protocols are important when handling permafrost specimens (for instance, the Russian labs take precautions when probing liquid blood from ancient bodies). Another angle is the slippery slope: will success with one species lead to attempts with more dangerous ones? Nobody is proposing reviving smallpox or Yersinia pestis (plague) from victims buried in permafrost, but ethicists mention scenario planning. In general, the consensus is that the pandemic risk from de-extinction is very low, but public fear might imagine “Ice Age diseases” running amok. Responsible communication and perhaps even regulatory oversight will be needed to address such fears and ensure safety protocols (for example, thoroughly screening any tissue cultures for pathogens).
Philosophical and Cultural Considerations
De-extinction also raises big questions about how humans relate to nature. If we resurrect species, do we “owe” them a particular kind of life? Some indigenous cultures have perspectives on extinct megafauna as part of their heritage; involving local communities (e.g., Yakutian Sakha people in Siberia or First Nations in Canada) in plans to reintroduce Ice Age animals is essential. There’s also something profound in seeing a lost animal returned – it could renew public interest in conservation through a sense of wonder. Shapiro has observed that the mammoth project, often likened to Jurassic Park, actually draws people into thinking about the current extinction crisis. “This is why kids are paying attention… if it takes people thinking ‘a real company is doing Jurassic Park’… then we’ve won”, she says, noting that it can make extinction and biotech front-of-mind for otherwise uninterested audiences. In that sense, even the idea of de-extinction serves as an educational tool and perhaps an inspiration to support science. However, this must be balanced against misperceptions – for example, the idea that we can “undo” any damage might lessen the urgency to protect what we have.
On the whole, the ethical debate is nuanced. Many scientists adopt a cautious but optimistic stance: they acknowledge the risks, yet see potential rewards. A 2017 Stanford University conference on de-extinction concluded that we should move from arguing if it’s right or wrong in theory to discussing how to do it responsibly. As California wildlife director Chuck Bonham said, “At some point we will be doing this… We need to stop worrying about theoreticals and start discussing how it will happen.”. That includes crafting guidelines for animal welfare, ecological assessments, and regulatory frameworks for any de-extinct releases.
Future Outlook: From Science Fiction to Reality
After years of speculation, de-extinction is entering a decisive decade. Advances in genomics, reproductive tech, and synthetic biology are converging to make the once impossible seem reachable. Colossal’s stated timeline of a 2027 mammoth birth may be optimistic, but it’s a sign of the field’s momentum (Popular Mechanics). Even if that deadline slips, steady progress – like the recent achievement of deriving elephant stem cells or finding intact chromosomal structures in a mammoth fossil – shows that scientists are methodically tackling each barrier.
By 2030 or 2040, we might well see the first creatures that haven’t walked the Earth in thousands of years, alive due to human technology.
It could start small: perhaps flocks of passenger pigeon hybrids winging over North American forests, or laboratory-grown thylacines living in large forested enclosures in Tasmania. And if the mammoth project succeeds, we may witness shaggy, rust-colored mammoth-elephants grazing the Siberian steppe once more. George Church envisions them eventually thundering across the Arctic, performing the ecological roles their ancestors did (Popular Mechanics). The impact on public imagination will be immense – it would be like bringing a piece of the Ice Age back to life.
Yet it’s important to temper the excitement with realism. De-extinction will not be a magic bullet to fix extinction or climate change. At best, it will be a complementary conservation tool. As Beth Shapiro emphasizes, the real promise of these technologies is to help extant species – the same methods needed to resurrect a mammoth (gene editing, IVF, etc.) are already being applied to save highly endangered species like the northern white rhino and to rescue populations vulnerable to disease or climate change genetically. In that sense, de-extinction research is driving innovation that benefits wildlife management broadly. Even if mammoths remain a bit “Jurassic Park”-like, the journey will yield valuable insights into genetics, developmental biology, and evolution. “The technologies one would need to bring back something similar to a mammoth are exactly what we need to preserve species that are still alive today,” Shapiro notes. This includes mastering genome editing for climate tolerance, optimizing cloning for fertility, and perhaps perfecting artificial wombs – all of which could aid species on the brink.
There’s also a likelihood that some projects will fail or be deemed too costly or unethical to continue. It’s possible that, for instance, no viable mammoth embryo is achieved in the next 20 years, or that societal opposition halts attempts to release any de-extinct animal into the wild. We must be prepared for setbacks and controversies. The first de-extinct animals will be under intense scrutiny, and any misstep (like an animal suffering or an ecosystem impact) could prompt a public backlash. Careful, transparent, science-driven approaches will be key to keeping de-extinction on a responsible path.
The rejuvenation of ancient frozen species is no longer a mere fantasy – it’s an active scientific quest blending paleontology and biotechnology. The permafrost has given us an unprecedented window into the past, preserving the very blueprints of extinct life. Now, teams around the globe are deciphering those blueprints and assembling the tools to build life from them. It’s a testament to human ingenuity – and perhaps, to our desire for redemption in the face of extinctions we’ve caused – that mammoths and cave lions are being contemplated for a second act on Earth.
Whether this grand experiment will succeed remains the big question. As one headline put it, “Maybe we shouldn’t resurrect the woolly mammoth”, to which others respond, “Why not? Are we that incurious?”. The coming years will provide the answer, as science navigates the tightrope between what we can do and what we should do. If done thoughtfully, the return of an Ice Age species could be an awe-inspiring triumph – a sign that we are entering a new era of planetary stewardship where extinction might not have to be forever.
Further Readings
- Church, G. interview in Harvard Medicine News; Business Insider; STAT Q&A with B. Shapiro; Revive & Restore quotes.
- Permafrost discoveries: ScienceAlert (Cassella 2023); The Archaeologist (2023); IFLScience (McCall 2017), iflscience.com; Smithsonian (Solly 2019).
- Cloning and genomes: NatGeo (Choi 2009); Sci-News (de Lazaro 2019); Popular Mechanics (Bennett 2016).
- Ethical and ecological commentary: Mongabay (DiGirolamo 2025); StatNews (St. Fleur 2024); Popular Mechanics (Newcomb 2023), Popular Mechanics; Smithsonian (Solly 2019).



