Hey, young explorers and future scientists! Have you ever wondered why your pasta cooks differently on a mountain than at home? Or why do mountaineers talk about their tea being lukewarm at high camps? It’s all about the boiling point of water and how it changes with altitude. Let’s dive into this steamy topic!
What’s the Boiling Point?
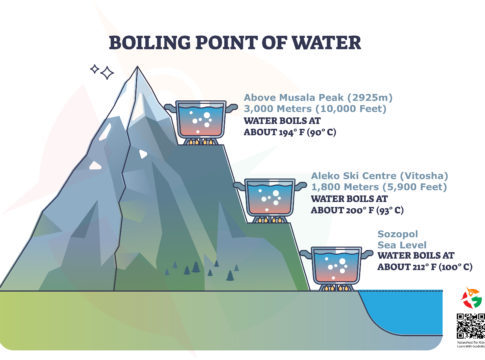
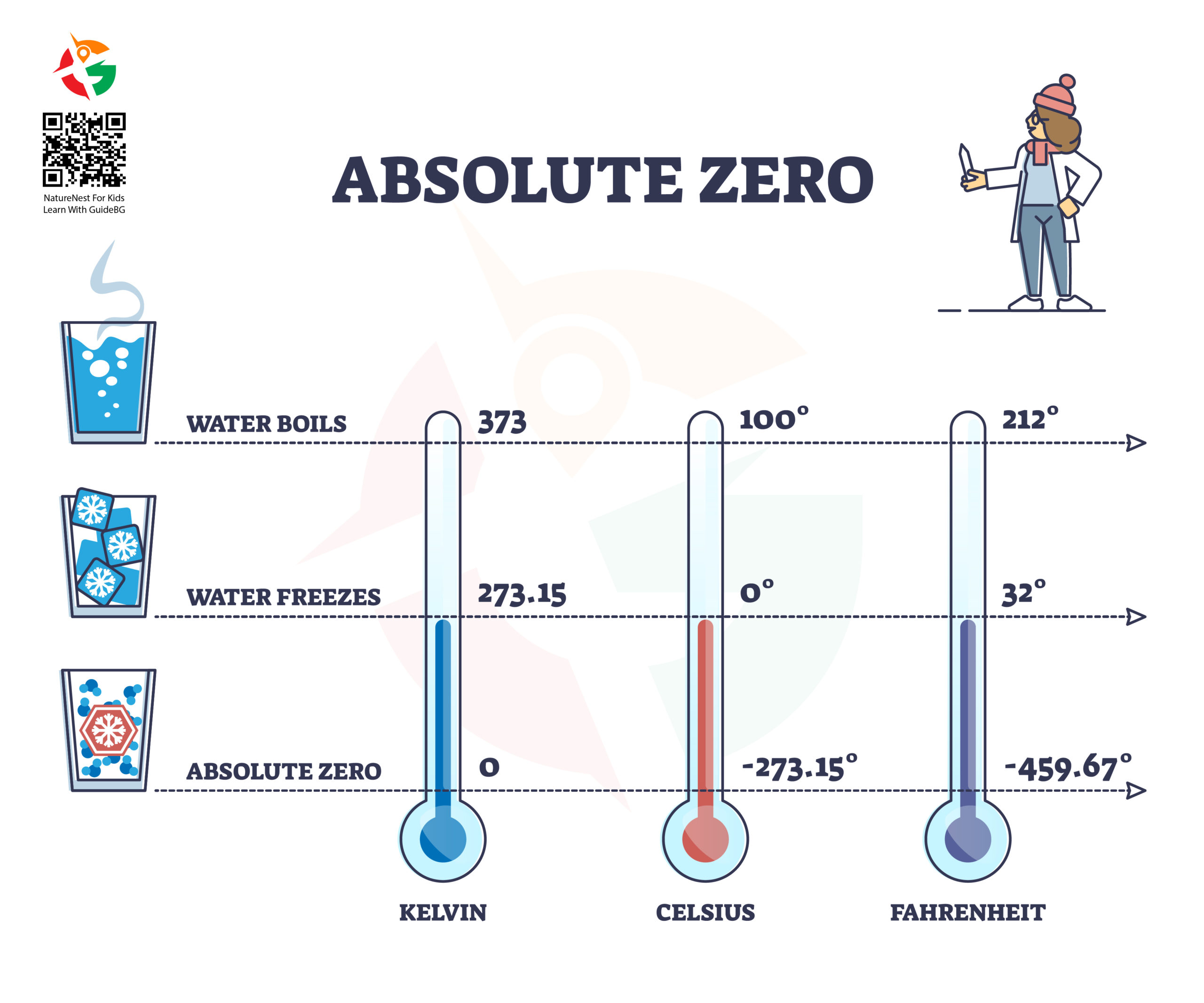
First up, the boiling point is the temperature at which water turns from liquid to vapor or, in simpler terms, starts to boil. At sea level, like in the coastal town of Sozopol, water boils at 100°C (212°F). That’s the magic number for cooking pasta perfectly or making tea.
High Up, Things Change
But, when you start climbing mountains, something interesting happens. The boiling point drops! This means water will boil at a lower temperature the higher you go. Let’s look at some examples:
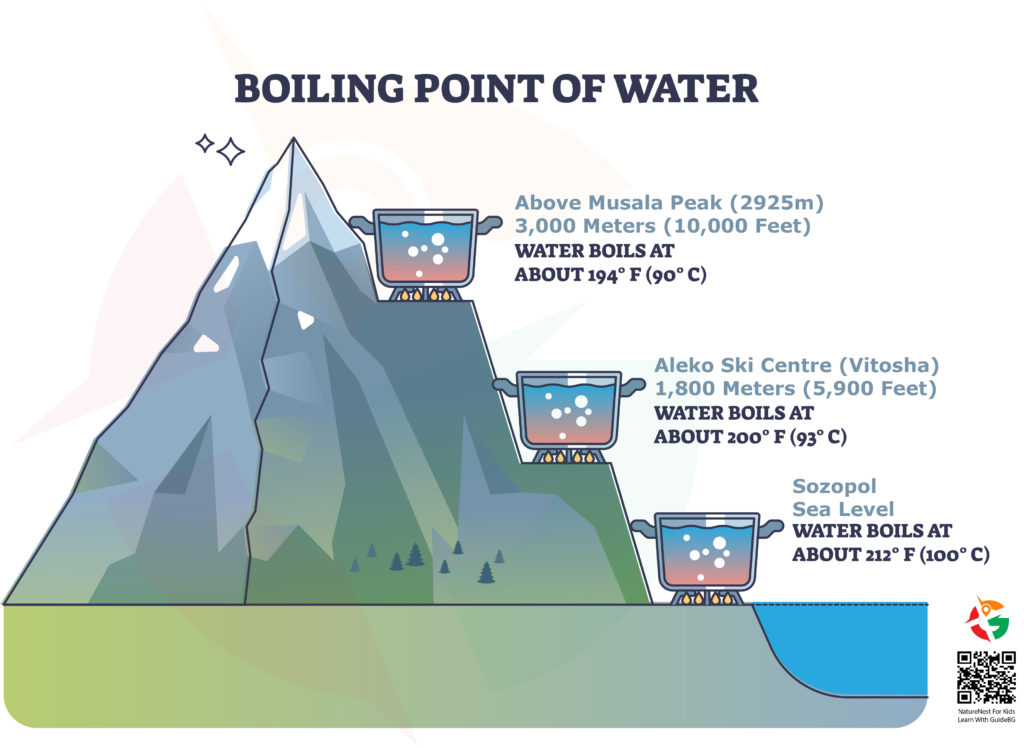
- At Sea Level (Sozopol): Water boils at 100°C (212°F).
- At 1,800 meters (5,900 feet) (Aleko Ski Centre), The boiling point falls to 93°C (200°F).
- At 3,000 meters (10,000 feet) above Musala Peak, It dips even further to 90°C (194°F).
Why Does This Happen?
The reason behind this fascinating phenomenon is air pressure. At sea level, the air is denser, pushing down on the water’s surface, which requires more heat for the water to boil. The air gets thinner as you climb higher, like reaching the heights above Musala Peak, the highest in Bulgaria. Less air pressure means it’s easier for water to turn into steam, so it boils at a lower temperature.
What Does This Mean for Mountain Adventurers?
It might take longer if you’re cooking or making a hot drink high up in the mountains, and the temperature won’t get as high. This can affect the taste and cooking time of your food. So, if you’re planning a high-altitude camping trip, remember your hot cocoa might not be as hot as you’re used to at home!
Understanding the boiling point of water isn’t just a remarkable science fact. It’s practical knowledge for outdoor enthusiasts and future scientists alike. So, remember the science behind those bubbles next time you’re boiling water, whether at the beach or atop a mountain. It’s all about altitude and how it affects the world around us. Keep exploring, keep questioning, and who knows what other everyday mysteries you’ll unravel!