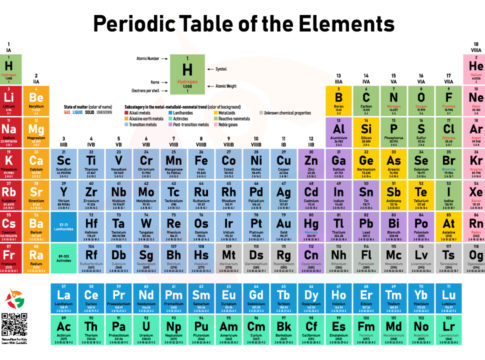
Hey, young scientists! Are you ready for another adventure into the enchanting realm of atoms and the Periodic Table? This chart isn’t merely a list; it’s the ultimate directory of elemental superheroes, the atoms that constitute everything around us. From the oxygen we inhale to the materials in our gadgets, every bit is represented in a spectrum of colors and symbols on this table. Let’s unravel this colorful guide and get acquainted with the families of atoms, the very elements that forge our universe.
Before diving deeper, let’s take a step back with our previous article, exploring the fundamental layout and fascinating trends that guide our journey through this elemental landscape.
The Elemental Families and Their Neighborhoods
Alkaline Metals (Red)
Starting on the left, in bold red, we find the Alkaline Metals, like Lithium (Li), Sodium (Na), and Potassium (K). They’re the first column of the table and are known for being super reactive and always ready for a chemical party.
Alkaline Earth Metals (Yellow)
Just next door in sunny yellow, the Alkaline Earth Metals stand firm. This family includes Calcium (Ca) and Magnesium (Mg), found in column two. They’re slightly more reserved than their Alkaline neighbors but still lively.
Transition Metals (Light Blue)
In the heart of the table, colored in light blue, the Transition Metals like Iron (Fe), Copper (Cu), and Gold (Au) show off their versatility. These elements are the artisans, crafting compounds and colors in endless combinations.
Lanthanides (Blue) and Actinides (Green)
These secretive siblings sit below the main table in shades of blue and green. The Lanthanides, like Neodymium (Nd), light up our tech world, while the Actinides, including Uranium (U), hold the power of radioactivity.
Post-Transition Metals (Purple)
We find the Post-Transition Metals in purple, like Tin (Sn) and Lead (Pb). These elements are the jack-of-all-trades, useful in many applications but less flashy than their Transition cousins.
Metalloids (Light Yellow)
Straddling the borderlands in light yellow, Metalloids like Silicon (Si) and Arsenic (As) blur the lines between metal and nonmetal, essential for semiconductors and mysteries alike.
Reactive Nonmetals (Dark Green)
The life-bringers, including Oxygen (O) and Carbon (C), are marked in dark green. They’re the storytellers, weaving tales of life and chemistry.
Noble Gases (Pink)
At the table’s edge in pink, the Noble Gases, such as Helium (He) and Neon (Ne), prefer solitude. They rarely react, holding their electrons close in an aura of mystery.
Elements with Unknown Chemical Properties (Grey)
In grey, some elements await discovery and understanding, and their chemical properties are still a question mark.
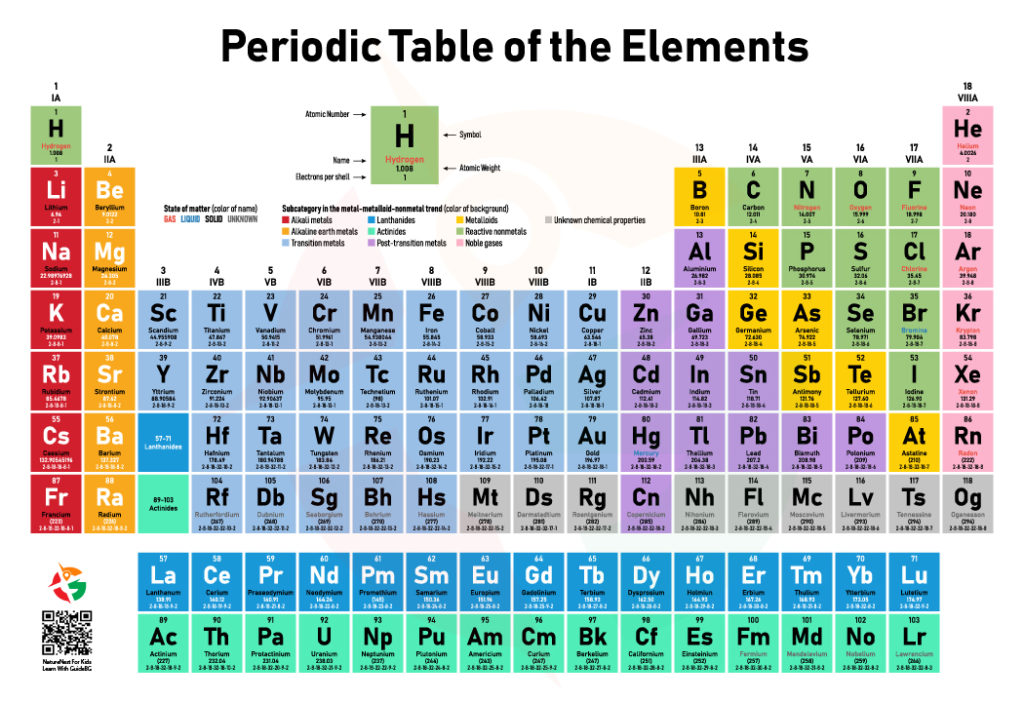
Decoding the Legend: Understanding Atom Basics
Every element on the Periodic Table comes with a legend, a mini-profile that tells you everything you need to know. Let’s use Hydrogen (H), the simplest atom, as our guide:
- Atom Symbol (H): The element’s abbreviation and superhero badge.
- Atomic Number (1): This tells you how many protons are in the atom’s nucleus. For Hydrogen, it’s the number 1, making it the first element.
- Atomic Weight (1.008): This number gives the average mass of the element, considering all its isotopes.
- Name: Just like a person, every element has a name. Hydrogen is the lightest and most abundant element in the universe.
- Electrons per Shell: This shows how the element’s electrons are arranged. Hydrogen has just one electron, riding solo in its shell.
The State of Matter: A Colorful Clue
The names of elements also come in colors to tell you their state of matter at room temperature:
- Red Names: These elements are gases, like Oxygen (O) and Nitrogen (N), floating free and unbound.
- Blue Names: These rare elements are liquids, such as Mercury (Hg), slinking and sliding in their metallic pools.
- Black Names: Most elements are solids, like Carbon (C) and Iron (Fe), strong and stable in their forms.
- Grey Names: For elements with unknown states, like some of the newest discoveries, mystery shrouds their form.
Embarking on an Elemental Adventure
Now that you know how to read the Periodic Table and understand the legend of elements, you’re ready to embark on countless adventures in the world of chemistry. Each element holds a story, from the fiery reactions of Alkaline Metals to the noble silence of Noble Gases.